TASC SCIENCE STUDY PACKET DAY 3: Earth and Space Science
Good Day TASC takers. Here is day 3 out of a 7 day science prep course. If you prefer to work offline and need to download the entire study file click on the link below:
Earth and Space Science Day 3: Use a model to describe how variations in the flow of energy into and out of Earth’s systems result in changes in climate.
Solar radiation (the process of giving off energy in the form of waves or particles) comes to Earth
mainly in the form of visible light and heat. Some of the light is reflected into space
when it hits Earth’s atmosphere. Some of the light is absorbed by the
atmosphere, while the remainder passes through the atmosphere and strikes Earth’s
surface. About half of the solar
radiation is absorbed on Earth’s surface, which warms up and radiates heat, or
infrared radiation, into Earth’s atmosphere. Some of the infrared radiation
is absorbed by the atmosphere while the rest passes through the atmosphere and
into space.
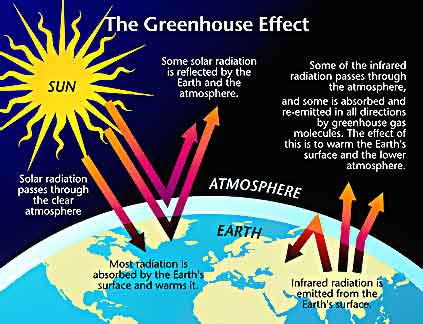
The amount of heat that is absorbed
by the atmosphere depends on the concentrations of greenhouse gases. A
greenhouse gas is a gas that is relatively transparent to visible light but
absorbs infrared radiation. The most
significant greenhouse gases in Earth’s atmosphere are water vapor (H2O), carbon
dioxide (CO2), and methane (CH4). Nitrous Dioxide (N2O), is another greenhouse gas that occurs naturally, but it has been increasing due to man made activities. The greater the concentration of greenhouse gases,
the greater the amount of infrared radiation that is absorbed by the atmosphere
and re-radiated down to Earth’s surface.


When CO2 is emitted into the atmosphere, much of it becomes dissolved in the oceans. While this “carbon sink” helps to slow the accumulation of CO2 in the atmosphere, it causes carbonic acid to form in the oceans, lowering the pH of seawater. Organisms such as corals, mollusks, echinoderms, crustaceans, and several types of plankton build shells of calcium carbonate. Normally, seawater is supersaturated with calcium and carbonate ions, making it relatively easy to build a shell. When the pH drops, the water becomes under-saturated, making it much harder to build and maintain a shell. Although the effects of ocean acidification are not fully understood, they represent yet another worry associated with global warming.

While surface temperatures have increased an average of only 1 °C worldwide, the effects of global warming have been observed in sensitive environments. Climate change caused by an asteroid impact is often accepted as the reason for the mass extinction of dinosaurs. Most recently we have seen climate change affect mountain glaciers as they have retreated (melted), sea ice in the Arctic Ocean has disappeared, permafrost in Siberia and Canada has melted, and the Greenland and Antarctic ice caps have shrunk. The carbon cycle is the chemical cycle by which carbon is exchanged among the biosphere (surface, atmosphere, and hydrosphere of the earth)of the Earth.
 |
| The Carbon Cycle: Notice that all carbon eventually ends up in the atmosphere. The ocean, soil, tree's, and the lithosphere store large amounts of carbon. |
Much of the current debate about global warming focused on the magnitude of positive and negative feedback loops that may accelerate or slow warming. For example, melting permafrost releases large quantities of methane into the atmosphere. Melting glaciers and sea ice expose darker materials that have a greater tendency to absorb light than reflective ice. Another important consideration concerning global warming deals with carbon sinks and there level of saturation. A carbon dioxide (CO2) sink is a carbon reservoir (storage) that is increasing in size, and is the opposite of a carbon "source." The primary natural sinks are oceans, plants, and other organisms that use photosynthesis to remove carbon from the atmosphere by incorporating it into biomass thereby affecting the rate of global warming.
We do not yet know how much saturation the worlds carbon sinks can absorb before it is too late for the environment to recover from. On the other hand, a hotter climate may intensify evaporation and increase the number of clouds. Clouds have a cooling effect because they reflect sunlight. Increased carbon dioxide may also spur plant growth. but scientist caution that there is also a saturation point that exists for plants as well. A major challenge for climate scientists will be to create mathematical models that accurately account for all of the complex interactions in Earth’s climate.
(Don't Worry This Is Not On The TASC!)
Day 3 Practice



Comments
Post a Comment